Beta-3 Integrins and Embryo Implantation
Implantation of embryos is a complex process. Initially, the embryo has to attach its placental cells to the surface cells of the uterine lining (the endometrium). This is a process that is mediated by a complex of proteins expressed both on the surface of the embryo and on the surface of the endometrium. Expression of the uterine proteins is under the influence of the ovarian hormone progesterone. There are estimated to be over 300 genes that are either turned off or turned on in the endometrium during the implantation window, the 3-4 days during which the endometrium is receptive to an embryo attaching. Most of the products of these genes and their role in implantation remain to be identified. In a small percentage of cases, failure to properly secrete one or more of these proteins may be a cause for implantation failure of normal embryos.
One protein produced by the endometrium during the implantation window that has some evidence for a scientific basis for a role in implantation is the cell-to-cell adhesion molecule known as beta-3 integrin. Integrins are a class of cell surface proteins that appear to act in all types of cell-to-cell recognition and adhesion processes. The beta-3 class of these proteins has been shown to be produced in response to progesterone in the endometrium and are purported to be one of the key proteins for adhesion of embryos to the endometrium. Failure to express this protein appropriately has been theorized to be a cause of unexplained implantation failure. Why some women do not produce beta-3 integrins is usually unknown. However, some proposed causes include presence of blocked fallopian tubes filled with inflammatory fluids (hydrosalpinx), endometriosis, and poor progesterone production.
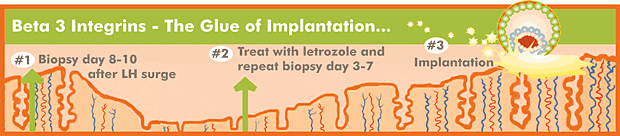
In order to diagnose whether or not a patient is producing beta-3 integrins, an endometrial biopsy must be performed 8-10 days after ovulation, as determined by LH surge testing. The biopsied endometrial sample is then sent to a laboratory that performs immuno-histochemical analysis on the tissue. The tissue is fixed to a slide and treated with antibodies to beta-3 integrins. These antibodies then are further treated with a second color marker antibody, so that endometrium-secreting beta-3 integrins will light up under the microscope. The tissue is scored by manual analysis by a medical technologist specifically trained to analyze beta-3 integrin expression.
In June of this year, I had the opportunity to visit Adeza Biomedical, a Cupertino-based laboratory that offers beta-3 integrin testing. I was impressed with the facility and the scientific integrity of the staff. I was also impressed with the labor-intensiveness of the analysis process. They receive specimens every day from infertility clinics across the country and are usually processing 6-12 specimens daily. They also send a portion of the biopsied tissue to a local pathologist to determine if the configuration (histology) of the endometrial tissue indicates it has been obtained within the implantation window or whether it is out-of-phase. As it turns out, a high percentage of tissue samples (40-45%) at Adeza are reported as negative for beta-3 integrins. A smaller percentage of these negative specimens are out-of-phase. So most of the specimens failing to show beta-3 integrins production are in-phase. It is unclear why this lab finds such a high rate of their test samples showing negative results for beta-3 integrins when the true incidence of lack of beta-3 integrins in most patients should be low. It may be that either the lab is setting the scoring level for a positive result too high or it may be that the patient samples really reflect a selected population of women who truly have low expression of beta-3 integrins. Unfortunately, there is no clear answer to this.
Previously, we had been less inclined to perform endometrial biopsies. Even if we found out there was a lack of beta-3 integrins, we wouldn't know what to do to induce their expression. However, we are beginning to find that we can often induce the expression by treating beta-3 integrin-negative patients with the aromatase enzyme inhibitor, letrozole (see A Closer Look at Letrozole; May 2006). Many women, especially if the histology on the original biopsy is in-phase, will have a positive biopsy result after treatment with letrozole.
Biopsies are typically performed 8-10 days after an LH surge in a natural cycle. Repeat biopsies on letrozole (taken days 3-7 of the cycle) are also performed at this time. We usually will use some local anesthetic in the cervix prior to passing a small plastic tube through the cervix to scrape out some endometrial tissue. Mild cramping may occur. The cost of the biopsy is $125.00 and the cost of the tissue analysis by Adeza is $400.00. It takes about 4-5 working days for the results to be received.
If you would like more information about this test, visit www.adeza.com and select the E-tegrity logo. You can download a patient brochure from this website. If you would like to know if this testing is appropriate for you, please ask your PFC physician. -- Carolyn Givens, M.D.
Categories
About the Blog
Welcome to the Pacific Fertility Center Blog! Nationally and internationally recognized for providing exceptional reproductive care, our team believes in empowering people with the knowledge they need to navigate their unique fertility journeys.
From information on the latest fertility treatments to valuable insights on egg donation, surrogacy, and everything in between, the Pacific Fertility Center Blog is your ultimate resource for all things reproductive care and support. Read on to learn more, and contact us today if you have any questions or want to schedule a new patient appointment.